How Do Mc1r Gene Mutations Cause Melanoma
MC1R codes for a seven-transmembrane G protein-coupled receptor expressed on the surface of melanocytes. It is a receptor for -melanocyte stimulating hormone and its antagonist, agouti.
The binding of -MSH to the cell membrane receptor on a melanocyte leads to a switch in melanin from the red/yellow phaeomelanin pigments to the brown/black eumelanin. The black eumelanin is photoprotective against the damaging effects of the suns ultraviolet radiation.
Mutations in MC1R can affect the change from phaeomelanin to eumelanin pigment and increase melanoma risk.
Braf Fusion Genes As A Novel Molecular Target
The classification of melanoma based upon the molecular profile of a patients tumor has changed the way melanoma is treated. While BRAF and MEK inhibitors in combination have become standard care for BRAF-mutant melanoma MEK inhibitors have activity in patients with NRAS-mutant melanoma . Multikinase inhibitors such as imatinib or sunitinib may have activity in KIT mutant melanoma however, the number of patients treated with these agents remains low, precluding detailed survival analysis. Preclinical data suggest that MEK inhibitors may be effective for patients with NF1 mutations . Approximately 30% of melanomas do not have clear driver mutations, and for these patients, the only available current treatments are immunotherapy and chemotherapy .
BRAF kinase fusions, occurring as a result of rearrangements that fuse the BRAF kinase domain to 5′ partner genes, have been shown to activate the MAPK pathway in several cancers including pilocytic astrocytoma, and gastric, thyroid and prostate cancers . In melanoma, BRAF fusions may occur in 48% of BRAF/NRAS/KIT wild-type patients . Similar to tumors with wild-type BRAF, type 1 RAF inhibitors appear ineffective and may paradoxically activate the MAPK pathway however, sensitivity to MEK inhibitors has been demonstrated in vitro .
Mek Inhibitors Combined With Braf Inhibitors For Metastatic Melanoma
The MEK gene has a close connection to the BRAF gene, so drugs that are targeted to MEK can also help treat melanoma with BRAF mutations. MEK inhibitors include trametinib , cobimetinib , and binimetinib .
The most common approach is to combine a MEK inhibitor with a BRAF inhibitor. This combination has been shown to be more effective than the use of either drug alone. When used together, BRAF and MEK inhibitors can shrink melanoma in the majority of people with BRAF-mutant melanoma.
Also Check: What Does Skin Cancer On The Outer Ear Look Like
Everyone Has The Braf Gene
BRAF is a gene that locks down a specific protein called B-Raf. This protein helps send signals inside your cells that are related to cell growth. Everyone has this gene, and when its working properly, its an important part of how cells operate. But when BRAF is faulty in some waysometimes known as changed or mutatedthen it might work against the body, by allowing some cancers to grow or even playing a role in that growth process.
Mutually Exclusive Mutations In Melanoma
- BRAF
- GNA11
- GNAQ
There is currently no recommendation for adjuvant therapy for class 2 patients, but this is an active area of research, she added.
The surveillance interval remains controversial. With early detection of oligometastatic disease, targeted intervention might be helpful, but this must be weighed against its cost and the risk of false-positives. Low-cost options for screening, such as liver function tests, have low sensitivity.
Because uveal melanoma almost exclusively goes to the liver, our algorithm for class 2 patients is surveillance every 3 months, generally alternating between and liver ultrasound, she said.
Focus on BRAF
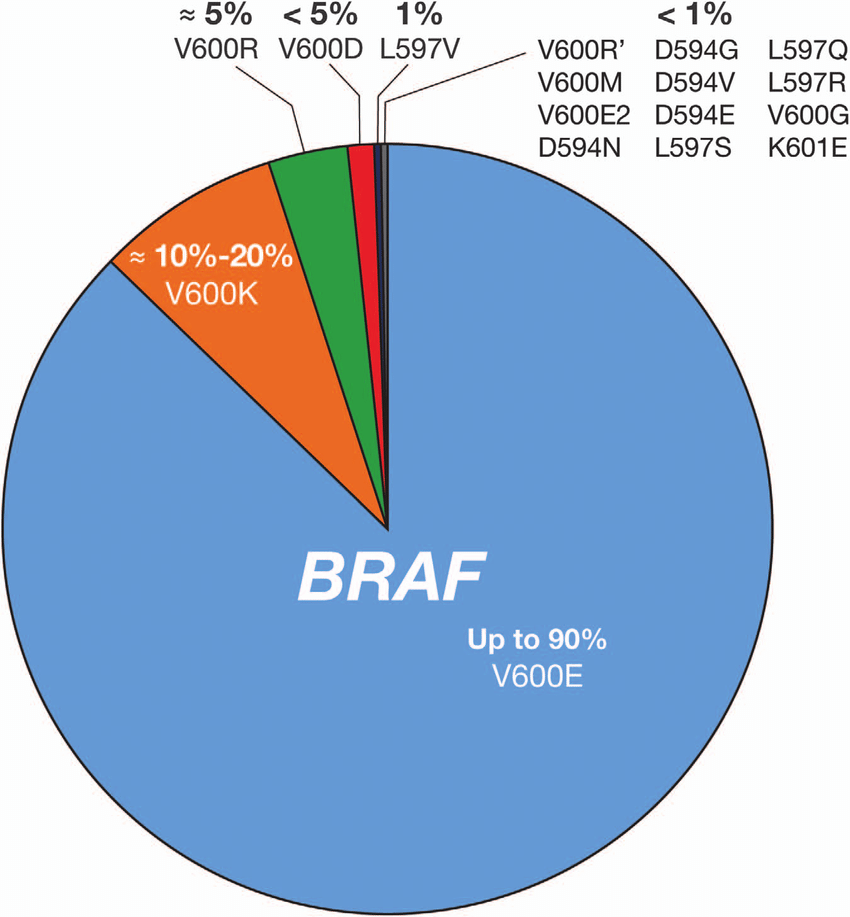
THE MOST CRITICAL mutation in melanoma is, of course, BRAF. More than 90% of BRAF mutations are located at codon 600 of them, more than 90% are at V600E about 5% are at V600K and a few are at V600R, V600E2, or V600D.
I would argue that all our metastatic melanoma patients should be tested foractionable mutations. Melinda L. Yushak, MD, MPHTweet this quote
Testing for BRAF mutations can change patient outcomes. There are different treatment options for patients with certain mutations, and some have shown improvements in overall survival, Dr. Yushak emphasized. Three BRAF/MEK inhibitor combinations are available for these patients: vemurafenib plus cobimetinib , and dabrafenib plus trametinib are approved by the U.S. Food and Drug Administration encorafenib plus binimetinib are in clinical trials.
Update on COMBI Trials
Also Check: How Long For Squamous Cell Carcinoma To Spread
Mutations Of The Braf Gene Are Related To Cancers
Although BRAF mutation and its connection to cancer was only identified in 2002, research done since then has pinpointed at least 30 different types of mutation that could be associated with cancers. The relationship between BRAF mutation and melanoma is particularly strong. The mutation doesnt play much of a role in other common cancers, such as lung cancer.
Role Of Genetic Testing To Determine Melanoma Risk
There are no widely accepted guidelines for managing families with an increased hereditary risk for developing cutaneous melanoma.
The American Society of Clinical Oncology has stated that screening genetic tests for CDKN2A and CDK4 have not yet been shown to be of medical benefit.
GenoMEL has published consensus statements recommending against routine genetic testing for familial cutaneous melanoma except in rare circumstances.
The rationale for their recommendations is as follows:
- mutations have not yet been detected in the majority of families with hereditary cutaneous melanoma
- our understanding of melanoma risk to carriers of gene mutations is limited
- other factors appear to influence the risk
- negative testing may provide false reassurance.
GenoMEL recommends that:
- all high-risk family members are trained in skin self-examination
- have baseline skin examinations at age ten years by a physician specialist
- continue clinical skin examinations every six months by a physician specialist until melanocyticnaevi are stable
- have annual or biannual examinations after that time depending on numbers of atypical naevi regardless of CDKN2A mutation status.
The lack of definitive evidence showing clinical benefit from genetic testing further supports continued strict surveillance and optimised solar protection as primary goals for prevention of malignant melanoma.
Recommended Reading: What Are The 4 Types Of Melanoma
The Mapk & Braf Mutations In Melanoma
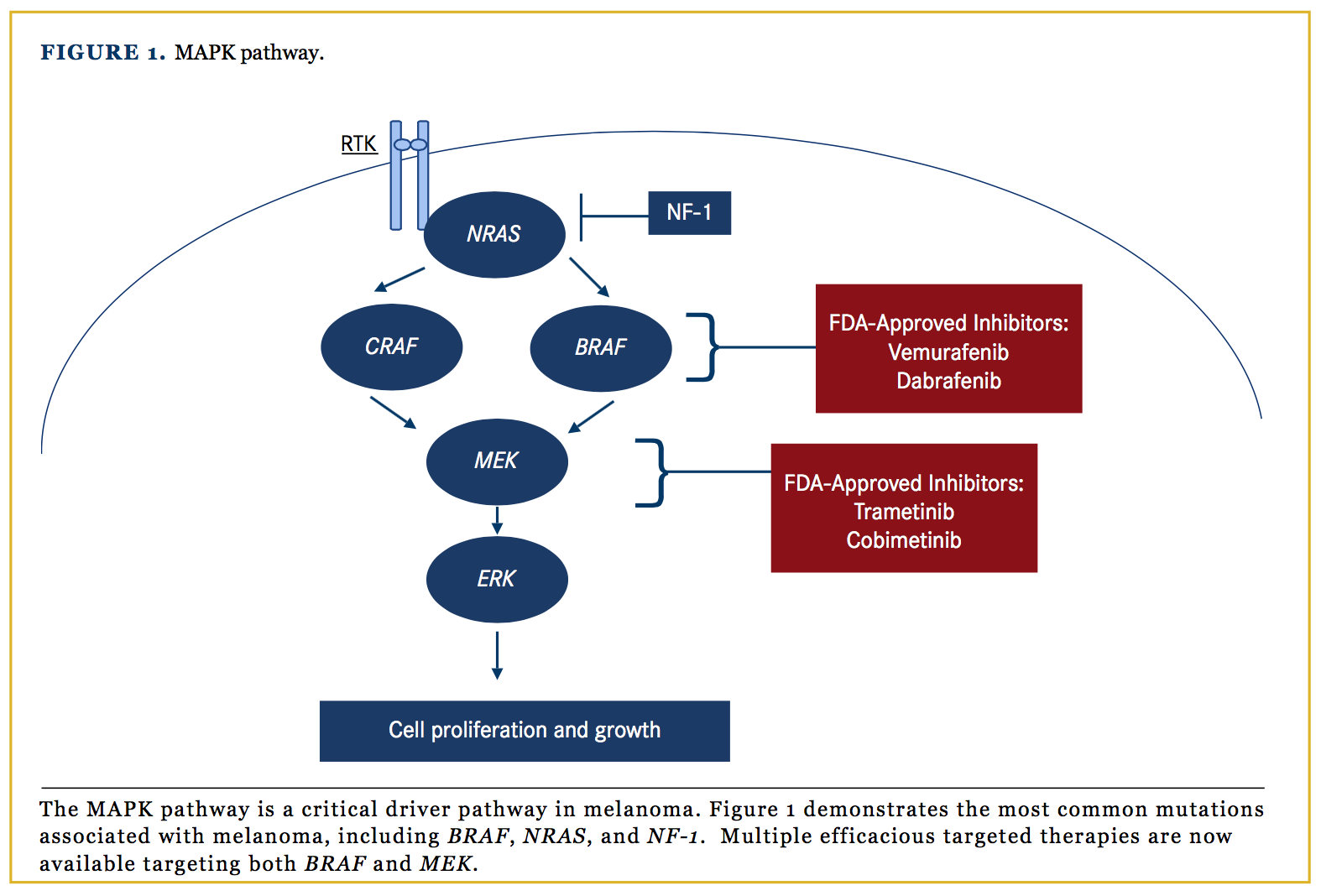
The MAPK pathway is one of the mechanisms by which basic cellular processes such as growth, proliferation and apoptosis are controlled . In normal cells, this pathway acts via extracellular signal-regulated kinase cascades, which ultimately control both cytoplasmic and nuclear targets involved in cellular proliferation in a highly regulated fashion, with feedback regulation by downstream elements. RAF is an upstream element of the MAPK pathway, sitting below RAS, and exists as three isoforms, ARAF, BRAF and CRAF. These cytoplasmic protein kinases are regulated by their interaction with RAS . Membrane-anchored RAS GTPases lead to homo- and heterodimerization and activation of the RAF family proteins, which subsequently activates MEK by phosphorylation. The PI3K pathway, which is another key cellular growth regulation pathway, is activated by RASGTP, representing a cross-talk mechanism between these two key pathways of cellular growth .
The MAPK pathway.
The MAPK pathway is overactive in the majority of melanomas, most often as a result of mutations in BRAF, NRAS and NF1 . The most prevalent driver in melanoma is mutant BRAF, found in 4050% of patients with metastatic disease. BRAF mutations occur in other cancers such as colon cancer, papillary thyroid cancer and serous ovarian cancer, but at a frequency much lower than in melanoma .
Braf Inhibitors For Metastatic Melanoma
Metastatic melanoma is cancer that has spread beyond its original location to other parts of the body. BRAF inhibitors are drugs that can shrink or slow the growth of metastatic melanoma in people whose tumors have a BRAF mutation. BRAF inhibitors include vemurafenib , dabrafenib ,and encorafenib .
MSK has taken a leadership role in the development of BRAF inhibitors for metastatic melanoma. In 2006, our doctors conducted the first clinical trial of vemurafenib, five years before the US Food and Drug Administration approved this treatment.
MSK experts are leading clinical trials to improve our ability to fight melanoma with BRAF inhibitors. Learn more about clinical trials for melanoma.
Recommended Reading: How Rare Is Merkel Cell Carcinoma
Optimal Sequencing Of Targeted Therapy & Immunotherapy
There is a paucity of data on the optimal sequence of targeted therapy and immunotherapy for BRAF mutant metastatic melanoma. Selected patients who have received ipilimumab will have a durable response regardless of whether they have residual disease or a complete response by RECIST . Although it is clear that a subset of patients treated with targeted therapy have durable survival, confounding factors such as crossover to other therapies in clinical trials have meant that this effect is difficult to measure . Long-term survival data for anti-PD-1 antibodies are awaited however, Phase I data for nivolumab have shown that the majority of patients have responses lasting at least 1 year .
The response rate to dabrafenib when used in first-line setting is 50%, whereas patients who crossed over to dabrafenib after disease progression had a response rate of 46% . Furthermore, the response rates across the Phase IIII trials of both vemurafenib and dabrafenib are similar, regardless of line of therapy, further suggesting that BRAF inhibitors have similar activity regardless of when they are used . In contrast to targeted therapy, available data suggest that that anti-PD-1 antibodies have the best activity in the first-line setting. Nivolumab monotherapy has an objective response rate of 40% in previously untreated patients, but following treatment with ipilimumab, the response rate is lower at 32% . Similar data exist for pembrolizumab .
Braf Mutational Assessment: State Of Art Of Companion Diagnostic And Laboratory
The BRAF mutational assessment landscape in melanoma patients has evolved over time, and the modern scenario is characterized by the availability of several companion diagnostic and laboratory-developed tests . Moreover, in most cases, a not negligible advantage is represented by abundant biopsy material for molecular tests, coming from a variety of primitive tumour excision or metastatic biopsies . Recently, the easily reproducible tool of liquid biopsy has also found applicability in melanoma patients, especially for monitoring therapeutic response . Indeed, it has been shown that in patients with advanced BRAF mutated melanoma undergoing treatment with TT, higher levels of plasma circulating tumour DNA may predict disease progression earlier than imaging and/or clinical assessments .
Recommended Reading: How Quickly Can Melanoma Appear
Braf May Play A Role In Other Cancers
Although its most associated with melanoma, the BRAF mutation may play a part in thyroid cancer, non-small cell lung cancer, and colorectal cancer. About 10 percent of colorectal cancer patients have a BRAF gene mutation, according to a report in Biomarkers in Cancer. Several clinical trials are ongoing to try and figure out the best treatment options for those with colorectal cancer who also have the BRAF mutation.
Targeting Braf Mutation In Melanoma Treatment
Melanoma is an aggressive disease with a poor prognosis when diagnosed at an advanced stage. Patients generally do not benefit from traditional chemotherapy but until very recently treatment options have been limited. In 2002, the discovery of a single-base substitution mutation in the BRAF gene that is common in malignant melanoma initiated the development of targeted therapy for the disease.1
BRAFis found downstream of NRAS in the mitogen-activated protein kinase pathway that aids in regulation of cell growth. Although BRAF mutations are seen in multiple cancers, the mutation predominates in melanoma activating BRAF mutations are found in 40% to 60% of melanoma tumors.1-5BRAF mutations and NRAS mutations, which are the second most common mutation found in melanoma, are considered mutually exclusive.3BRAF mutations occur at early stages of the disease and are preserved through metastatic transformation.3
While there are multiple pathways to cell growth, BRAF-mutated tumors rely entirely on MAP kinase pathway activation such that BRAF inhibition checks tumor growth.4 This insight led to clinical development of small molecule kinase inhibitors designed to block BRAF. Without BRAF inhibitor treatment, the presence of a BRAF mutation is associated with poor prognosis for patients diagnosed with stage IV melanoma. When these patients receive treatment with a BRAF inhibitor, their prognosis is better than that of stage IV patients lacking the mutation.4
| Drug |
You May Like: What Are The Types Of Skin Cancer
Braf Mutations Other Than Or In Addition To V600
Regarding BRAF mutational status, all four of the described cTT trials only included patients harboring a BRAF V600E/K mutation, yet less frequent BRAF mutations have been described. Recently, Menzer et al. analyzed the clinical outcomes of 103 patients harboring rare BRAF mutations or translocations . Of these patients, seven had been excluded from statistical analysis due to a co-existing BRAF V600E/K mutation. Of the remaining 96 patients, 58% received BRAFi/MEKi combination therapy 76% of the patients were treatment-naïve, whereas the remainder had prior treatment that mostly consisted of immunotherapy and chemotherapy. Elevated LDH levels at baseline were found in 42% of patients. The assessed 96 patients were split into two groups, depending on a BRAF V60 mutation and BRAF mutations affecting other codons or BRAF chromosome translocations resulting in a mutational activation of BRAF. OS for patients harboring a V600 mutation was 17.3 months , compared with 11.3 months for non-V600 mutations if treated with cTT. The median PFS was 8.0 months for V600 mutations and 3.3 months for non-V600 mutations. The ORR was 56% and 28%, respectively. Although only a small number of patients were included, activating mutations in BRAF outside of codon 600 seem to be associated with decreased efficacy of cTT.
Table 2 Baseline characteristics affecting the PFS of cTT or ICB in pivotal trials
Vemurafenib: Phase I And Ii Results
In phase III studies, dacarbazine, the only chemotherapeutic agent approved by the U.S. Food and Drug Administration for the treatment of metastatic melanoma, was associated with a response rate of 7 to 12 % and a median overall survival of 5.6 to 7.8 months after the initiation of treatment.
Recently a selective and potent inhibitor of oncogenic mutant BRAF , vemurafenib , gave positive results in phase I and phase II trials 2) .
The structural formula of vemurafenib.
The phase I study was a multicenter, 55-solid cancer patients dose-escalation trial followed by a 32-melanoma patients extension phase involving the maximum dose that could be administered without adverse effects . Vemurafenib was administered at a starting dose of 160 mg daily and was generally well tolerated with no dose-limiting toxicity until the 720 mg twice-daily dose level was initiated. This trial demonstrated that vemurafenib has very impressive single-agent clinical activity, with unprecedented objective response rates in about 81 % and a confirmed response rate in about 56%of patients who had melanoma with the BRAFV600E mutation the study also showed a clear impact on PFS > 7 months and established the maximum tolerated dose to be 960 mg twice-daily. Responses were observed at all sites of disease, including the bone, liver and small bowel. During the dose escalation phase, responses were also observed in patients who were receiving doses below the recommended one .
Read Also: What Does In Situ Melanoma Mean
Pi3k/akt/mtor Pathway And Pten
As well as the MAPK pathway, the phosphoinositide-3-kinase /protein kinase B /mammalian target of rapamycin signalling pathway is involved in cellular growth, proliferation and survival . PI3K, stimulated by membrane receptors, converts, through its catalytic domain, phosphatidylinositol -bisphosphate on the plasma membrane into phosphatidylinositol -trisphosphate , which is the docking site for AKT. After its binding, AKT can activate mTOR complex , leading to protein synthesis and cellular proliferation by stimulating 4E binding protein 1 and ribosomal protein S6 kinase 1 . Dysregulation of this pathway is frequent in metastatic melanoma however, mutations of genes involved in this pathway are rare , meaning that other molecular mechanisms can activate PI3K-AKT signalling, i.e., NRAS, c-KIT and HER4 activating mutations . Loss of phosphatase and tensin homolog , which catalyses dephosphorylation of PIP3 in PIP2 thus inactivating the PI3K pathway, has been detected in no more than 30% of melanoma patients intriguingly, loss of PTEN is never associated with NRAS mutations in melanoma, whilst it is frequent in BRAF-mutant ones . PTEN loss was associated with a non-statistically significant shorter PFS in BRAF-mutant melanoma patients treated with BRAF inhibitor a recent work affirmed that PTEN-negative melanoma patients have poor outcome as the result of resistance to both TT and ICIs .
Current State Of Target Treatment In Braf Mutated Melanoma
- 1Medical Oncology, IRCCS Ospedale Policlinico San Martino, Genoa, Italy
- 2Genetics of Rare Cancers, IRCCS Ospedale Policlinico San Martino, Genoa, Italy
- 3Genetics of Rare Cancers, Department of Internal Medicine and Medical Specialties, University of Genoa, Genoa, Italy
Incidence of melanoma has been constantly growing during the last decades. Although most of the new diagnoses are represented by thin melanomas, the number of melanoma-related deaths in 2018 was 60,712 worldwide . Until 2011, no systemic therapy showed to improve survival in patients with advanced or metastatic melanoma. At that time, standard of care was chemotherapy, with very limited results. The identification of BRAF V600 mutation, and the subsequent introduction of BRAF targeting drugs, radically changed the clinical practice and dramatically improved outcomes. In this review, we will retrace the development of molecular-target drugs and the current therapeutic scenario for patients with BRAF mutated melanoma, from the introduction of BRAF inhibitors as single agents to modern clinical practice. We will also discuss the resistance mechanisms identified so far, and the future therapeutic perspectives in BRAF mutated melanoma.
Read Also: What To Do When You Get Skin Cancer
Braf Inhibitors Plus Mek Inhibitors
Preclinical studies suggested that the addition of a MEK inhibitor to a BRAF inhibitor could reduce the side effects of BRAF inhibitor as single agent , delay the development of resistance and generate a synergistic improvement in efficacy outcomes . On this wave, several studies have been performed. Taken together data from these clinical trials demonstrated a statistically significant superiority of the combination compared to monotherapy in terms of OS, PFS and ORR .
Dabrafenib plus Trametinib. The phase I/II trial demonstrated the safety of dabrafenib and trametinib combination and its significant superiority in terms of ORR and PFS over dabrafenib monotherapy, among patients with BRAF V600E/K-mutated, unresectable or metastatic melanoma. Moreover, a subsequent survival analysis showed its advantage in terms of survival . Based on these results, the combination of dabrafenib and trametinib received FDA approval in January 2014, with an accelerated procedure.
The phase III trial, COMBI-d compared dabrafenib plus trametinib with dabrafenib monotherapy. In the primary analysis, median PFS and ORR were significantly increased in the combination arm. A subsequent update demonstrated that the combination reduced the risk of death by 29% compared with monotherapy with a 3 years OS of 44 vs 32% .