What Is Advanced Scc
When a squamous cell carcinoma of the skin has spread extensively or aggressively, or has resisted multiple treatments and repeatedly recurred, it is considered to be advanced.
These tumors include:
- Locally advanced SCC: Tumors that are large or have penetrated deep into underlying tissues, muscles or nerves. These SCCs can be disfiguring and/or can compromise these underlying structures.
- Metastatic SCC: Tumors that have spread beyond the original location to other parts of the body. These SCCs can be life-threatening.
If youve been diagnosed with advanced SCC, your doctor may recommend an evaluation by a multidisciplinary team to explore treatment options. The team may include your dermatologist and/or Mohs surgeon, along with physicians and surgeons from other specialties. After surgery to remove the tumor and, if necessary due to metastasis, local lymph nodes, options may include a combination of treatments, based on the complexity of the disease and your overall health. The regimen can include:
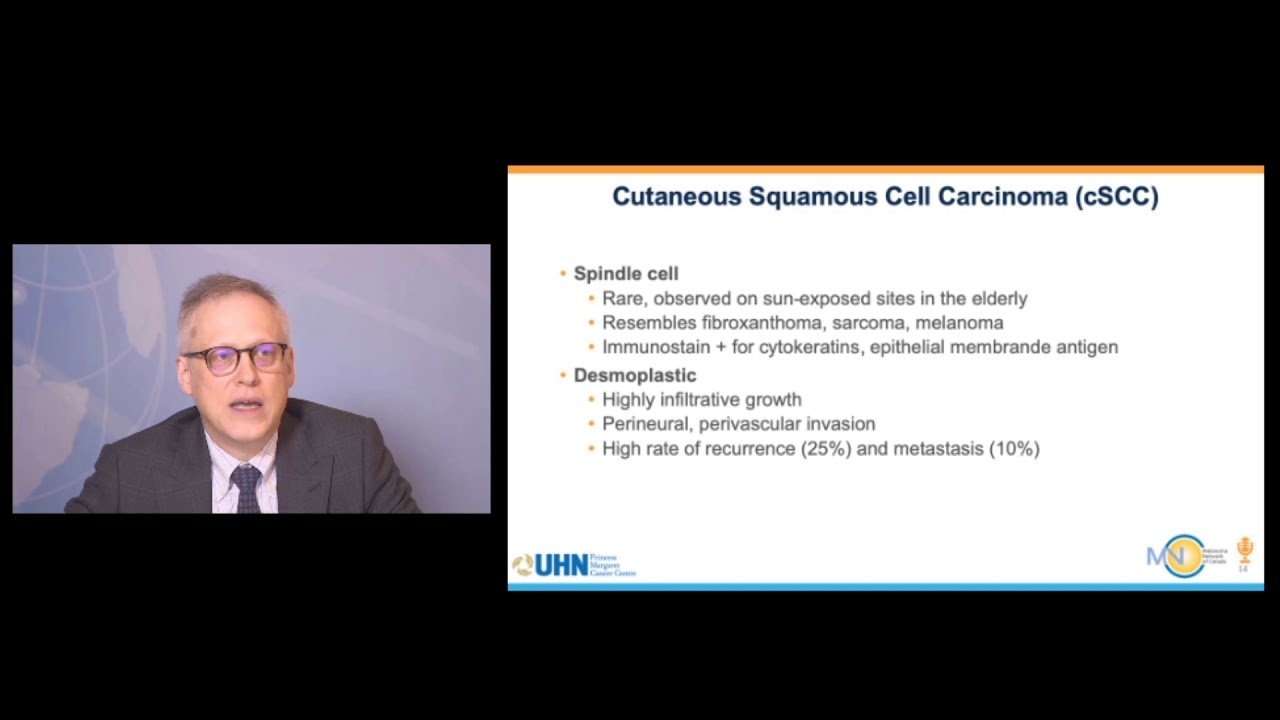
Not Your Typical Squamous Cell Carcinoma
The second most common non-melanoma skin cancer, cSCC is usually treated with local therapies by dermatologists. However, aggressive cSCC requires more extensive resection and radiotherapy. Medical and radiation oncologists treat this aggressive disease, which often appears on the head and neck area, similarly to head and neck squamous cell carcinoma .
CSCC does not behave like traditional mucosal head and neck squamous cell carcinomas, says Dr. Geiger. Though the way we treat them is similar to other head and neck cancers resection, followed by radiation and potentially systemic therapy cSCC is a different disease with a different biology, and the patient population tends to be different than mucosal HNSCC.
Until recently, there were no systemic therapies approved for cSCC by the U.S. Food and Drug Administration . That changed in 2018 when PD-1 inhibitor cemiplimab was approved to treat metastatic and other advanced cSCC in patients who were not candidates for resection or radiation.
We know immunotherapy works in cSCC, says Dr. Geiger. But can it be used with resection and radiation to prevent recurrence or metastasis? Thats what KEYNOTE-630 will help us determine.
Recurrence or metastasis occurs in 40-50% of patients with high-risk, locally advanced cSCC.
Targeting Two Signaling Pathways
Long-lasting infections with certain types of HPV can cause cancers of the cervix, throat, anus, rectum, penis, vagina, and vulva. Recent research on the biology of HPV-related cancers supports the strategy of targeting both PD-L1 and TGF-beta, according to Dr. Gulley.
Increased levels of PD-L1 have been found in HPV-related cancers. And TGF-beta is often present in the tumor microenvironment of HPV-related cancers and may play a role in the growth, progression, and spread of tumors.
TGF-beta also appears to play a role in preventing immune cells from infiltrating tumors, said Dr. Gulley. Reducing the amount of TGF-beta in the tumor microenvironment may enable immune cells to do their jobs and attack tumor cells, he added.
Maura L. Gillison, M.D., Ph.D., of the University of Texas MD Anderson Cancer Center, who discussed the trial during the AACR session, agreed. Its reasonable to expect that targeting TGF-beta may work against HPV-related cancers and may augment the effects of inhibiting a checkpoint protein, she said, noting that the new findings appear to support this view.
The overall survival results from the trial are extremely promising, Dr. Gillison added.
A phase 2 trial now under way is testing the drug in a larger group of patients with HPV-related cancers, including patients who have not received immune checkpoint therapy or have progressed during treatment with checkpoint therapy, according to Dr. Strauss.
Recommended Reading: Basal Skin Cancer Survival Rates
Keytruda For Hodgkin Disease
Keytruda is FDA-approved to treat classical Hodgkin lymphoma . This type of lymphoma is a cancer that develops in your B cells. And its the most common form of Hodgkin lymphoma.
For this condition, Keytruda is given to adults with refractory or relapsed cHL. With refractory cHL, the cancer either doesnt improve with treatment or doesnt stay improved after treatment for very long. And with relapsed cancer, it comes back after being treated in the past.
Keytruda is also used in children to treat cHL that has relapsed after two or more past treatments.
Keytrudas effectiveness for Hodgkin disease
In a clinical study, Keytruda was effective in treating cHL. In the study:
- 47% of people taking Keytruda had their cancer partially go away
- 22% of people taking Keytruda had their cancer completely go away
- of people whose cancer either partially or completely went away, half of them kept these results for at least 11.1 months
Keytruda For Kidney Cancer

Keytruda is FDA-approved to treat advanced renal cell carcinoma . For this condition, its approved for use in combination with either axitinib or lenvatinib .
Keytruda can be given as a first-line treatment to adults with advanced kidney cancer.
Keytrudas effectiveness in kidney cancer
In one study, Keytruda was effective in treating kidney cancer when used in combination with axitinib . In the clinical study, people taking Keytruda with axitinib had better overall survival* and progression-free survival** compared with people taking a cancer drug called sunitinib .
At 12 months of treatment, 90% of people taking Keytruda with axitinib were still alive. In comparison, 78% of people taking sunitinib without Keytruda were still alive at 12 months. Half of the people taking Keytruda with axitinib also had a progression-free survival of at least 15.1 months. In comparison, half of the people taking sunitinib had a progression-free survival of at least 11.0 months.
* Overall survival describes the length of time people lived after they joined the study. ** Progression-free survival describes the length of time that people lived with their disease without the disease getting worse.
Don’t Miss: Can Squamous Cell Carcinoma Metastasis
Other Systemic Therapies Being Studied
A number of clinical trials are ongoing with investigational agents for squamous cell skin cancer. Other FDA-approved PD-1 inhibitors , such as nivolumab, pembrolizumab, and avelumab, are being studied in squamous cell skin cancer. As mentioned previously, studies are also ongoing with targeted therapies alone and in combination with chemotherapy agents.
Treating Squamous Cell Skin Cancer: Treatment Options
Once you have gone over the pathology report and staging information with your doctor, its time to plan the treatment strategy. This section discusses the different types of therapy that are available for squamous cell skin cancer and their advantages and disadvantages.*
These treatments are applied directly to your skin to treat squamous cell skin cancer.
Don’t Miss: Etiology Of Basal Cell Carcinoma
Financial And Insurance Assistance
If you need financial support to pay for Keytruda, or if you need help understanding your insurance coverage, help is available.
Merck Sharp & Dohme Corp., the manufacturer of Keytruda, offers a program called The Merck Access Program. For more information and to find out if youre eligible for support, call 855-257-3932 or visit the program website.
Combining Targeted Therapy And Immunotherapy
To examine the efficacy of ICB in the SA model, we randomized SA males with established PSCC at 45 month old to receive isotype IgG or an anti-PD1/anti-CTLA4 antibody cocktail at doses described recently. The treatment lasted for one month and affected minimally on tumor weight . To enhance ICB, we focused on two FDA-approved drugs, cabozantinib and celecoxib. Cabozantinib is a multi-targeted tyrosine kinase inhibitor and recently shown to block PI3K signaling in MDSCs to enable efficacy from ICB in metastatic prostate cancer. Celecoxib is a selective COX2 inhibitor and highly relevant to our study, because Cox2 was upregulated in the SA tumors and identified as the top putative master regulator for inflammation-related phenotypes . Cyclooxygenase-dependent tumor immune evasion is through myeloid cell reprograming and Cox2 blockade with celecoxib synergizes with anti-PD1 or dendritic cell immunotherapy in syngeneic models of a few cancer types,.
Fig. 4: Combined targeted therapy and immunotherapy for spontaneous PSCC in mice.
Read Also: What Is The Most Aggressive Skin Cancer
Icis For Recurrent And Metastatic Scchh
The treatment strategy for RM-SCCHN is determined according to the sensitivity to platinum agents. Platinum-naïve or progressive diseases more than 6 months after curatively intended platinum-based chemotherapy given for locoregionally advanced disease are designated as platinum-sensitive. On the other hand, progressive diseases during or within 6 months after platinum-based chemotherapy are designated as platinum-resistant.
The EXTREME trial showed that adding cetuximab, an anti-EGFR monoclonal antibody, to chemotherapy with carboplatin or cisplatin and 5-fluorouracil increased overall survival in patients with platinum-sensitive RM-HNSCC. Thus, platinum-based chemotherapy plus cetuximab was a standard of care for platinum-sensitive RM-SCCHN . Therapeutic options for platinum-resistant cases in clinical practice included taxane, methotrexate, S-1 and cetuximab monotherapy, if not chosen in the first line. The organizational framework for this review is structured around platinum sensitivity.
Transcriptomic Analysis Of Mouse Penile Cancer
Fig. 2: Transcriptomic analysis reveals activation of -catenin signaling and inflammatory pathways in mouse PSCC.
a Hierarchical clustering of differentially expressed genes between WT and SA mouse penile samples . b Gene regulation network by -catenin , the top upstream regulator identified by IPA to account for differential gene expression changes in SA tumors compared with WT penis. Red and green colors indicate upregulation and downregulation, respectively. ce IHC stain for -catenin, Sox2 and Cox2 in WT and SA penis, respectively. Scale bar 20m for c, 100m for d, 200m for e. f Mechanistic network for the top ranked master regulator Ptgs2/Cox2 by IPA to illustrate its effect on cytokine expression regulation and function in immune cells. g Western blot showing differential protein expression in penile tissues from WT, PB-Cre4+Smad4L/L and SA mice. h IHC stain for Ki67 in SA penile tumor. Scale bar 200m. i Sox2 expression silenced by two independent shRNA in SA1 cell line, detected by western blot. j Growth curves of subcutaneous tumors formed by control or Sox2 knockdown sublines of SA1 in nude mice . k, l Weight and gross images of subcutaneous tumors formed by control or Sox2 knockdown sublines of SA1 at endpoint . In j, k, data represent mean±SD. **P< 0.01, ****P< 0.0001, two-sided Students t test.
Read Also: Does Insurance Cover Skin Cancer Screening
Ongoing Recruitment In 19 Countries
Recruitment started in March 2019 and is underway in 19 countries.
Our goal is 570 patients, so it will require multi-institutional involvement since advanced cases of cSCC are relatively rare, says Dr. Geiger. We look forward to collecting sufficient data that we expect will show that pembrolizumab provides a durable response in these patients.
For more information about this trial, see clinicaltrials.gov/ct2/show/NCT03833167.
Image credit: National Cancer Institute
Side Effects In Children
The side effects of Keytruda seen in children are similar to those seen in adults. However, in a small clinical study, children who took Keytruda had higher rates of certain side effects than did adults using the drug.
Side effects of Keytruda that were more common in children than in adults included:
- fatigue
- elevated liver enzymes
If you have questions or concerns about side effects in children using Keytruda, talk with your pharmacist or your childs doctor.
Recommended Reading: Amelanotic Melanoma Blanch
Keytruda For Esophageal Cancer
Keytruda is FDA-approved to treat squamous cell esophageal cancer and gastroesophageal cancer in certain cases.
Esophageal cancer develops in squamous cells found on the surface of your esophagus. Gastroesophageal cancer forms in the area where your stomach meets your esophagus.
Keytruda treats these types of cancer that cant be treated with surgery or a combination of chemotherapy and radiation. And the cancer must be either:
- locally advanced , or
- metastatic
Keytrudas effectiveness for esophageal cancer
In clinical studies, Keytruda was effective in treating esophageal cancer. In one clinical study, people taking Keytruda had better overall survival compared with people taking chemotherapy .
For example, half of the people taking Keytruda had an overall survival of at least 10.3 months. In comparison, half of the people taking chemotherapy had an overall survival of at least 6.7 months.
Immunology Of Head And Neck Squamous Cell Carcinoma
The immune system modifications noted in HNSCC patients suggest that this cancer is an overall immunosuppressive process. In the peripheral bloodstream, HNSCC patients have less overall number of white blood cells, which are comprised of a greater proportion of suppressive regulatory T cells . Additionally, TIL within HNSCC tumors are comprised of an even more suppressive population of Treg cells than in the peripheral bloodstream of HNSCC patients .
Human papillomavirus positive HNSCC tumors have one of the higher levels of infiltrating Tregs. Studies exploring the relationship of Treg infiltration to patient prognosis are varied some show improved prognosis with a higher number of TIL Treg, , and others showing this benefit only with a high CD8/Treg ratio as seen with HPV+ disease . High CD8+ TIL seen in HPV+disease has been shown in several studies to confer improved disease-free survival . These cell populations express IR that can be targeted by inhibitory checkpoint receptor blockade therapy .
Recommended Reading: Does Skin Cancer Make You Lose Hair
Rationale Of Using Icis In Head And Neck Cancer
HNSCC is an appropriate disease for immunotherapy, as immune escape plays a key role in its tumor initiation and progression. Several groups have examined the expression of PD-L1 in human SCCHN tissue samples across multiple primary sites, and these studies revealed high levels of PD-L1 expression, on 46100% of tumors . Therefore, T-cell checkpoint inhibitors blocking the PD-L1:PD-1 association have been evaluated targeting RM-SCCHN.
The majority of SCCHN cases carry a high mutational burden, which is probably linked to heavy smoking, and the resultant tumor neoantigen may be a target of the hosts immune system. Subgroups of oropharyngeal cancer and nasopharyngeal cancer are associated with human papillomavirus and EpsteinBarr virus , respectively. HPV-positive cancers might have mutations attributable to the expression of APOBEC cytidine deaminases . The expression of viral proteins, such as EBV nuclear antigen-1 or latent membrane proteins 1 and 2, can elicit a virus-specific immune response in patients with nasopharyngeal cancer .
Keytruda For Cervical Cancer
Keytruda is FDA-approved to treat cervical cancer. This form of cancer develops in the cervix, which separates a womans uterus and vagina.
Keytruda is used to treat cervical cancer that has high levels of the immune system protein called PD-L1.
Its given in combination with chemotherapy, with or without bevacizumab , to treat cancer thats either:
- persistent
- recurrent
- metastatic
Keytruda is used by itself to treat recurrent or metastatic cervical cancer that has worsened either during or after chemotherapy.
Keytrudas effectiveness for cervical cancer
In one clinical study, Keytruda was effective in treating cervical cancer. In one study:
- 11.7% of people taking Keytruda had their cancer partially go away
- 2.6% of people taking Keytruda had their cancer completely go away
- of people whose cancer either partially or completely went away, 91% kept these results for at least 6 months
Also Check: Stage 3 Basal Cell Carcinoma Survival Rate
The Old Therapeutic Options
Regarding EGFR inhibitors, there are limited data in the curative and postoperative setting. Specifically, postoperative cetuximab concurrent with radiotherapy versus radiation therapy alone in patients with high-risk head and neck CSCC , showed an advantage in terms of both freedom from local recurrence and freedom from distant recurrence . In the advanced setting, a phase II study including 36 patients with CSCC showed a response rate of 28% with a median duration of response of 6.8 months . Similar results were also observed with dacomitinib, with grade 3-4 adverse events being observed in 36% of patients, and 16% of patients discontinuing treatment because of drug-related toxicity . Finally, in a large retrospective case series, both chemotherapy and targeted therapy for the treatment of advanced CSCC showed response rates of less than 20%, with overall survivals of less than 20 months .
In summary, these treatment approaches were unsatisfactory, both in their impact on survival and quality of life , and a standard regimen for the treatment of advanced CSCC was not clearly defined, with up to 60% of patients with locally advanced CSCC not receiving any systemic therapy at all .
Will My Doctor Order A Lab Test To Check Whether Keytruda Is Right For Me
Yes, they will most likely do that. Keytruda is used to treat certain types of cancer that have high levels of a protein called PD-L1. This protein is found on some cancer cells and on some healthy cells.
Some peoples cancer cells have more PD-L1 protein than other peoples cancer cells do. Because Keytruda works to block this protein, its used to treat cancers that have more of the protein.
If you have certain types of cancer, your doctor will order a test for you to see if you have elevated levels of PD-L1.
Some types of cancer have unique traits other than PD-L1 that help Keytruda work on them. For example, some solid tumors or colon cancers have genetic mutations that make Keytruda a good treatment choice.
Before your doctor prescribes Keytruda, theyll check any tests that are needed to make sure its the right drug for you.
The following information is provided for clinicians and other healthcare professionals.
You May Like: Does Skin Cancer Burn And Itch
Keytruda For Liver Cancer
Keytruda is FDA-approved to treat hepatocellular carcinoma.* This type of cancer is the most common form of liver cancer.
Keytruda is approved to treat hepatocellular carcinoma in people whove taken the cancer drug sorafenib in the past for this condition.
* For this use, Keytruda received from the FDA. Accelerated approval is based on information from early clinical trials. The FDAs decision for full approval will be made after additional clinical trials are completed.
Keytrudas effectiveness for liver cancer
In a clinical study, Keytruda was effective in treating liver cancer in people whose cancer either worsened or came back after treatment with sorafenib . And Keytruda was effective for this use in people who couldnt tolerate Nexavars side effects.
In the study:
- 16% of people taking Keytruda had their cancer partially go away
- 1% of people taking Keytruda had their cancer completely go away
- of people whose cancer either partially or completely went away, 89% kept these results for at least 6 months
- of people whose cancer either partially or completely went away, 56% kept these results for at least 12 months